Prediction of severe COVID-19 courses avoids fatal consequences and reduces costs
The Crit-CoV-U study
The Crit-CoV-U study to predict severe COVID-19 progression from day "0" of SARS-CoV-2 infection with over 1,000 patients is published in the scientific journal The Lancet Digital Health since August, 31st 2022!
The paxlovide drug - approved to prevent severe COVID-19 courses - is rarely given or not given in time!
Paxlovide is only effective in the first few days. In these first days, no clinical symptoms of a severe COVID-19 course are recognisable. Because of the not inconsiderable side effects of paxlovide, a dilemma arises that cannot be solved by the attending physician without diagnostic help. Especially since a preventive administration of paxlovid is excluded by its marketing authorisation.
The xCoV test from protexam ends the dilemma
The protexam xCoV is independent of the SARS-CoV 2 variant. It works for any strain because the test is based exclusively on endogenous human biomarkers.
From day "0" of the COVID-19 infection (PCR test), the protexam xCoV test determines the severe course to the hard endpoint "death" with a prognostic validity of over 80%. The previously known parameters, such as the WHO score, do not provide a prognosis, only the current status.
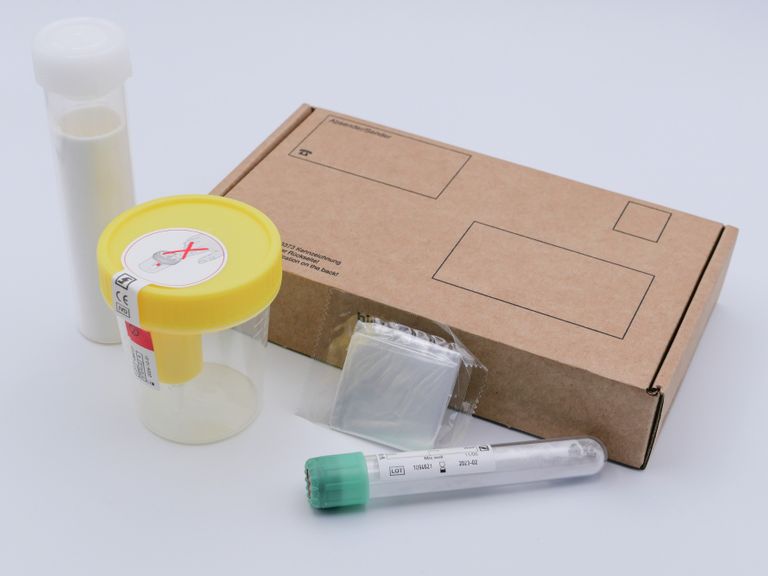
protexam xCoV test
The result of the protexam xCoV test will be sent to the attending physician within 1-2 days after receiving the urine sample.
Ask your doctor for a urine collection tube with stabiliser (borate powder). The universal test kit (including return shipping kit) can be sent to you for a fee of 85€ so that you have it on hand in case you are infected. Within max. 12 hours, depending on where you live, the sample will be in the laboratory after being handed over to the shipping company.
Protection against new variants
Virus variants can acquire a variety of mutations, for example when immunocompromised individuals experience long-term infections. This could also be a threat for future variants under certain circumstances.
There are often no adapted vaccines for the newer variants of SARS-CoV-2.
Whether these variants are less harmful than other previous mutations is difficult to predict for the colder months.
Where is the efficient protection?
As of mid-September 2025, Germany is experiencing an uptick in COVID-19 cases, consistent with trends seen in other parts of the world.
A recent study suggests that COVID-19 poses a higher disease burden than influenza, with more hospitalizations and deaths, and a more severe course of disease.
Who is at risk of severe disease with COVID-19?
As a rule, these are all chronically ill people and especially immunosuppressed patients. Around 30 million people in Germany alone are chronically ill. A significant proportion of the chronically ill are unthinkable.
Damage to the edothelium and fibrosis are identified by renowned physicians as the disposition for a possible severe course. The clinical proteome tests by protexam also identify these pre-existing conditions at an early stage. Because of this comprehensive proteomic analysis, the Crit-CoV-U study was able to successfully determine the high prognostic validity of over 80% from day "0" with included patients aged 18 and over.

Cost savings of up to €90,000 per patient
The consistent use of the protexam xCoV test relieves the intensive care units and saves lives! A scientific simulation of the possible savings effects was carried out based on the costs of the German healthcare system, which has now been published in addition to the data from the more than 1,000 patients from the Crit-CoV-U study. Early therapy reduces hospital days (5-10 days) and costs per patient day between €1,200 and €4,500.
According to Wirtschaftswoche, the costs for an average patient in the intensive care unit are between €5,000 and €92,000.
History of the development of the xCoV test
Temporal outline of the events
2020 Spring: BMG calls for biomarkers for the SARS-CoV-2 pandemic.
2020 May: Pilot study with 15 patients (published in Sept. 2020)
2020 June: Funding of the prospective, multi-centre CRIT-CoV-U study on the prognosis of the severe course of COVID-19 by the BMG after examination by the BfArM.
2020 November: After just a few patients, there is an outstanding recognition of the severe course on day "0" of the Covid-19 diagnosis.
2020 December: BfArM issues a special approval for the test after a further edition of 80 blinded patients and extensive examination of the sub-study with 327 patients.
2021 January: Regular approval of the test.
2021 February: Partial study with 327 patients is made available online on THE LANCET preprint server for timely testing of faster patient care.
2021 April: In the draft bill, the BMG provides for the reimbursement of the proteome test for those with statutory health insurance at the expense of the statutory health insurance companies. This fails due to the refusal of the three members of the G-BA with voting rights and the statutory health insurance companies. They claimed that only the results of the pilot study with 15 patients were available.
2021 May: Publication of the sub-study of the CRIT-CoV-U study in the form of special approval for 327 patients.
2021 August: Submission of the full report on 1,012 patients from the completed CRIT-CoV study to BfArM and BMG.
2021 November: 700 million worth of monoclonal antibody drugs against severe Covid-19 courses are disposed of. They were not awarded, even though 300 patients died from Covid-19 every day. The drugs are only effective in the first few days, when there are no symptoms of the severe course.
2022 January: German Bundestag instructs the Health Committee to examine the inclusion of the test in the national test ordinance.
2022 January 25: Publication of the complete CRIT-CoV-U study on preprint server: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4006139
2022 February: BMG agrees to purchase the active ingredient Paxlovid to treat severe Covid-19 courses.
2022 April: Paxlovid drugs are under-allocated. 250 patients die of Covid-19 every day.
2022 June: General Practitioners' Association points out the side effects of Paxlovid and that it is not medically justifiable to administer it prophylactically without symptoms and rejects the BMG's demand for more widespread use.
2022 August 31: Publication of the results of the CRIT-CoV-U study with 1,012 patients, which detects the severe course of Covid-19 from day "0" of the PCR determination to the hard endpoint with over 80% prognostic validity and provides evidence of a cost reduction the use of the test.
The kidneys, the most filigree organ in the human body - 1.5 million filters per kidney filter 1,700 liters a day, form 180 liters of primary tap and excrete only 1.5 liters. The kidney the seismograph of the body.
20 years of research
Utility of clinical proteome analysis
For the protexam test, the patient's proteome (entity of proteins) from the blood filtrate is evaluated using clinical proteome analysis (CE-MS coupling). The proteome patterns of the diseases, validated in clinical studies, are detected from up to 20,000 proteins fragments. This is done with AI support from an individual raw data set of the patients of 6 GB. In 20 years of research by around 400 renowned physicians, 100 clinical studies and over 400 high-ranking scientific publications have been made possible. They are all based on Prof. Harald Mischak invented CE-MS technology to collect clinically relevant proteomes. Today, for the first time, doctors and patients can recognize diseases at the molecular level, which is the only place where diseases arise and where drugs only work efficiently, e.g. in kidney and heart diseases, as well as in tumors.